Association Between Marijuana Use and Adverse Obstetrical and Neonatal Outcomes
- PMID : 26401751
- DOI : 10.1038/jp.2015.120
Abstract and Introduction
Abstract
Objective: To evaluate associations between marijuana exposure and adverse outcomes excluding women with polysubstance abuse and stratifying for concurrent maternal tobacco use.
Study Design: We performed a retrospective cohort study evaluating various obstetrical and neonatal outcomes including: preterm delivery, pre-eclampsia, gestational diabetes, cesarean delivery, fetal growth restriction, a composite which included stillbirth or neonatal intensive care unit admission, and perinatal mortality. We stratified study groups according to the maternal tobacco use and performed a logistic regression analysis.
Results: We included 6468 women, 6107 nonusers and 361 marijuana users. After adjustment for maternal age, race, parity, body mass index and no prenatal care, we found higher rates of small for gestational age (aOR 1.30 (95% CI 1.03 to 1.62)) and neonatal intensive care unit admission (aOR 1.54 (1.14 to 2.07)) in women who were not tobacco users. Other obstetrical outcomes including preterm delivery and fetal anomalies were not increased with maternal marijuana use.
Conclusion: Maternal marijuana use does not increase the risk of adverse obstetrical outcomes or fetal anomalies, but does increase the risk for small for gestational age and neonatal intensive care unit admission.
Introduction
As of the publication of this manuscript, 23 states and the District of Columbia have legalized the use of marijuana in some form, four states have passed legislation legalizing the recreational use of marijuana, and others are considering legalization of marijuana.[1] In 2012, the Drug Enforcement Agency (DEA) reported 18.9 million Americans aged 12 years or more used marijuana within the last month.[2] According to the DEA it is anticipated that with the legalization of marijuana and a heightened perception of acceptance of use, the incidence of reported use will also increase, especially among adolescents. By multiple measures available on the DEA website, marijuana use among the young is dramatically increasing already, with 27% of teens using marijuana within the last month, an increase of 47% since 2008. With the increasing use of marijuana in young adults, rate of exposure during pregnancy is also likely to increase. Finally, in addition to the increasing incidence of use, there has been an increase in the potency of marijuana with increases in the concentration of the active ingredient in marijuana, tetrahydrocannabinol to just under 15%, as compared with 4% in the 1980s.[2] This is likely contributing to both increases in adverse effects of use and addiction potential.
The active ingredient in marijuana is delta-9-tetrahydrocannabinol which readily crosses the placenta and produces high levels of carboxyhemoglobin.[3] In addition, delta-9-tetrahydrocannabinol is fat soluble and therefore has a long half-life, on the order of 6 days. Although marijuana use is both common and increasing in women of reproductive age, there are few studies exploring the adverse effects of marijuana use in pregnancy and inadequate or conflicting data on whether marijuana use is associated with higher rates of fetal anomalies, growth restriction (FGR), prematurity and other adverse obstetrical and neonatal outcomes.[3–9] The complex interplay between marijuana use, polysubstance use, concurrent tobacco use and adverse perinatal outcome is unknown. The goal of our study was to examine the associations between marijuana use and selected adverse obstetrical and neonatal outcomes, excluding women with polysubstance use and stratifying for concurrent tobacco use.
Methods
We performed a retrospective cohort study of all singleton deliveries born over 20 weeks at the University of Cincinnati Medical Center between January 2008 and January 2011. This study was approved by the Institutional Human Subjects Review Committee. All data were derived directly from review of the prenatal and delivery medical record. Women were excluded from our analysis if they had a multiple pregnancy or polysubstance use.
Marijuana users were designated as such if they reported use during the course of their prenatal care or at the time of delivery, or if at any point during the pregnancy they had a positive toxicology screen for tetrahydrocannabinol. Universal drug screening was not used during the study period, but was performed in pregnancies deemed to be high risk for substance abuse, secondary to known history of substance abuse, poor prenatal care or social/medical risk factors for drug abuse. The entire medical record was reviewed, and if prenatal records, laboratory results and the inpatient records revealed no evidence of use, patients were classified as nonusers.
The goal of this study was to examine a large cohort of women for associations between perinatal use of marijuana and various obstetrical and neonatal outcomes. The study was not powered to analyze any one particular outcome, and therefore is underpowered for rarer adverse outcomes, such as stillbirth and perinatal mortality. Adverse obstetrical outcomes studied included preterm delivery <37 weeks, gestational diabetes, pre-eclampsia, abruption, fetal growth restriction (FGR) defined as a sonographic estimation of fetal weight less than the 10th percentile, induction of labor, cesarean delivery after labor attempt, delivery for nonreassuring fetal status and/or oligohydramnios and stillbirth. We evaluated neonatal outcomes such as major fetal anomaly (a significant anomaly noted on the delivery admission history that required neonatal evaluation or intervention), small for gestational age (SGA) (birth weight less than the 10th percentile for gestational age), neonatal intensive care unit (NICU) admission, prolonged neonatal length of stay over 3 days, and perinatal mortality defined as either a stillbirth or death prior to infant discharge from the nursery or NICU.
Data were collected and managed using Redcap electronic data capture tools posted at the University of Cincinnati.[10] Continuous variables were compared using Wilcoxin rank-sum analysis. Chi-square analysis was used to compare categorical variables. Given there were in total 14 outcomes analyzed, Bonferroni correction would determine significance as a P-value of 0.004.
Logistic regression was performed to assess associations between marijuana exposure and selected obstetrical and neonatal outcomes. Tobacco was deemed to be a strong confounding variable and therefore we performed a stratified logistic regression analysis in tobacco users and nonusers. Backward selection was used to determine which potential confounding variables had a significant association with the outcomes of interest, those without impact were sequentially removed from the model. After step-wise elimination maternal age, race, parity, BMI (body mass index) class and late care/no prenatal care were included in the final regression model. When analyzing the outcomes ‘NICU admission’ and ‘neonatal length of stay over 3 days’ gestational age at delivery and presence of a fetal anomaly were also included in the regression model given known confounding. Odds ratios (OR) and adjusted odds ratios (aOR) for outcomes of interest were calculated and were presented with 95% confidence intervals. All data analysis were performed using NCSS 8 statistical software (Release 8. NCSS LLC, Kaysville, UT, USA).
Results
We managed 6841 deliveries over the study period: 178 patients were excluded for multiple pregnancy and 205 were excluded for polysubstance use. After considering exclusion criteria, 6468 were included in our analysis: marijuana nonusers, n=6107 (94.4%) and marijuana users, n=361 (5.6%). An additional two women were excluded from our post hoc analysis given that we were unable to determine if they were tobacco users or nonusers. Marijuana users tended to be slightly younger than nonusers, 24 (5.2) years versus 25.3 (5.9) years (mean (s.d.)) for users and nonusers, respectively, P<0.001 (). In addition, Hispanic and ‘other’ ethnicities reported significantly less marijuana use. As expected, marijuana users had significantly higher rates of concurrent tobacco use, 58% versus 20% in nonusers, P<0.001. Marijuana users had clinically similar gestational ages at presentation for prenatal care (15(10) weeks versus 16(9) weeks in nonusers and users, respectively, P=0.04), rates of obesity (31.7% versus 28.4%, P=0.21) and rate of no prenatal care (4.4 and 6.4%, P=0.08).
Table 1. Maternal demographic data
| Non-users (n=6107) | Marijuana users (n=361) | P-value | |
|---|---|---|---|
| Demographic data | |||
| Maternal age, mean±s.d. | 25.3±5.9 | 24±5.2 | <0.001 |
| Race (%) | <0.001 | ||
| White | 1786 (94) | 103 (6) | |
| Black | 3091 (92) | 253 (8) | |
| Hispanic | 1047 (99) | 1 (0.1) | |
| Other | 183 (98) | 4 (2) | |
| Maternal obesity, n (%) | 1788 (31.7) | 95 (28.4) | 0.21 |
| Tobacco use, n (%) | 1214 (20) | 208 (58) | <0.001 |
| GA at first visit (weeks), mean±s.d. | 15±10 | 16±9 | 0.04 |
| No PNC, n (%) | 294 (4.4) | 23 (6.4) | 0.08 |
| GA at delivery, mean±s.d. | 37.6±3.4 | 37.3±3.7 | 0.27 |
Abbreviations: GA, gestational age; PNC, prenatal care. Obesity is defined as body mass index over 30 kg m− 2.
We did not find increased risks of several adverse obstetrical outcomes in marijuana users versus nonusers including preterm delivery <37 weeks (20% versus 23%, P=0.15), pre-eclampsia (9% versus 7%, P=0.12), stillbirth (1.1% versus 1.5%, P=0.54) or unplanned cesarean delivery (32% versus 33%, P=0.75) (). Marijuana users had a lower rate of gestational diabetes (7% versus 4%, P=0.04) and a lower rate of induction (20% versus 25%, P=0.04), which was considered not significant after Bonferroni correction.
Table 2. Obstetrical and neonatal outcomes in marijuana nonusers versus users
| Nonusers (n=6107) | Marijuana users (n=361) | aOR (95% CI) | P-value | |
|---|---|---|---|---|
| Obstetrical complications, n (%) | ||||
| Preterm delivery | 1212 (20) | 83 (23) | 1.04 (0.91–1.19) | 0.15 |
| Induction | 1495 (25) | 71 (20) | 0.88 (0.77–1.01) | 0.04 |
| Cesarean delivery after labor | 1947 (32) | 118 (33) | 1.03 (0.88–1.21) | 0.75 |
| Gestational diabetes | 407 (7) | 14 (4) | 0.87 (0.66–1.04) | 0.04 |
| Pre-eclampsia | 572 (9) | 25 (7) | 0.84 (0.68–1.04) | 0.12 |
| Abruption | 89 (1.5) | 8 (2.2) | 1.17 (0.81–1.70) | 0.25 |
| FGR | 172 (2.8) | 14 (3.9) | 1.14 (0.86–1.51) | 0.24 |
| Stillbirth | 73 (1.1) | 6 (1.5) | 1.03 (0.62–1.72) | 0.54 |
| Neonatal complications, n (%) | ||||
| Anomaly | 218 (3.4) | 12 (2.9) | 1.04 (0.77–1.40) | 0.62 |
| SGA | 648 (11) | 67 (19) | 1.31 (1.13–1.51) | <0.001 |
| NRFS/oligohydramnios | 259 (4.2) | 22 (6.1) | 1.24 (0.98–1.57) | 0.09 |
| NICU admission | 752 (12.5) | 61 (17.2) | 1.37 (1.12–1.66) | 0.01 |
| Perinatal mortality | 158 (2.6) | 14 (3.9) | 1.24 (0.87–1.77) | 0.14 |
| LOS >3 days | 846 (14) | 60 (17) | 1.12 (0.95–1.31) | 0.14 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; FGR, fetal growth restriction; LOS, length of stay; NICU, neonatal intensive care unit; NRFS, nonreassuring fetal status; SGA, small for gestational age. After Bonferroni correction, P<0.004 would be considered statistically significant. Post hoc analysis controlling for maternal age, race, parity, BMI class and no prenatal care; aOR and 95% CI are presented. For the outcomes NICU admission, perinatal mortality and neonatal LOS >3 days model also included gestational age at delivery and presence of fetal anomaly.
Marijuana use was associated with selected adverse neonatal outcomes. Marijuana users had higher rates of SGA versus nonusers, 19% versus 11%, P<0.001. Marijuana users had higher rates of NICU admission for the neonatal, 17.2% versus 12.5%, P=0.01, which was not statistically significant after Bonferroni correction. Finally, perinatal mortality was higher in marijuana users compared with nonusers (3.9% versus 2.6%, P=0.14), which did not reach statistical significance given underpowered for this variable. Of note, marijuana users had a similar rate of major fetal anomaly (2.9%) as nonusers (3.4%), P=0.62.
Logistic regression demonstrated an association between marijuana use and SGA, aOR 1.30 (95% CI 1.03 to 1.62) and NICU admission, aOR 1.54 (95% CI 1.14 to 2.07), in non-tobacco users even when controlling for potential confounding variables (). We found a strong trend toward an association with prolonged neonatal hospital stay, aOR 1.21 (95% CI 0.96 to 1.53) and perinatal mortality in marijuana users who did not smoke, aOR 1.44 (95% CI 0.97 to 2.14). However, in women who also used tobacco the additional marijuana exposure did not substantially increase these neonatal risks (Figure 1) over tobacco use alone. Marijuana use was not associated with any other adverse obstetrical or neonatal outcomes after post hoc analysis in either concurrent tobacco users or in tobacco nonusers.
Table 3. Logistic regression analysis comparing marijuana users versus nonusers (referent) for selected obstetrical and neonatal outcomes stratified by concurrent tobacco use
| Marijuana nonusers (n=153) | Marijuana users (n=4892) | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Tobacco nonusers (n=5045) | ||||
| Preterm delivery | 932 (19.1) | 33 (21.6) | 1.08 (0.89–1.31) | 1.09 (0.89–1.33) |
| Gestational diabetes | 319 (6.2) | 9 (5.9) | 0.95 (0.67–1.33) | 1.16 (0.82–1.65) |
| Pre-eclampsia | 454 (9.3) | 12 (7.8) | 0.91 (0.68–1.23) | 0.95 (0.70–1.29) |
| Abruption | 67 (1.4) | 1 (0.7) | 0.69 (0.26–1.85) | 0.72 (0.27–1.94) |
| Fetal anomaly | 164 (3.4) | 9 (4.6) | 1.18 (0.80–1.73) | 1.29 (0.87–1.92) |
| FGR | 132 (2.7) | 4 (2.6) | 0.98 (0.59–1.63) | 0.99 (0.60–1.64) |
| SGA | 468 (9.6) | 25 (16.3) | 1.36 (1.09–1.69) | 1.30 (1.03–1.62) |
| Stillbirth | 52 (1.1) | 2 (1.3) | 1.11 (0.55–2.26) | 1.16 (0.57–2.38) |
| NICU admission | 575 (12.0) | 27 (18.1) | 1.27 (1.03–1.58) | 1.54 (1.14–2.07) |
| Perinatal mortality | 126 (2.6) | 7 (4.6) | 1.35 (0.91–1.99) | 1.44 (0.97–2.14) |
| LOS >3 days | 658 (13.5) | 27 (17.6) | 1.17 (0.95–1.45) | 1.21 (0.96–1.53) |
| Marijuana nonusers(n=1200) | Marijuana users (n=223) | |||
| Tobacco users (n=1423) | ||||
| Preterm delivery | 280 (23.1) | 50 (24.0) | 1.02 (0.86–1.22) | 1.04 (0.87–1.24) |
| Gestational diabetes | 88 (7.2) | 5 (2.4) | 0.56 (0.36–0.89) | 1.04 (0.87–1.24) |
| Pre-eclampsia | 118 (9.7) | 13 (6.3) | 0.78 (0.59–1.06) | 0.81 (0.60–1.10) |
| Abruption | 22 (1.8) | 7 (3.4) | 1.37 (089–2.12) | 1.37 (0.88–2.12) |
| Fetal anomaly | 40 (3.3) | 10 (4.8) | 0.85 (0.53–1.36) | 0.91 (0.56–1.46) |
| FGR | 40 (3.3) | 10 (4.8) | 1.22 (0.85–1.74) | 1.23 (0.86–1.76) |
| SGA | 180 (14.8) | 42 (20.2) | 1.21 (1.00–1.45) | 1.17 (0.96–1.41) |
| Stillbirth | 13 (1.1) | 2 (1.0) | 0.95 (0.45–2.00) | 1.00 (0.47–2.12) |
| NICU admission | 177 (14.8) | 34 (16.6) | 1.07 (0.88–1.31) | 1.29 (0.96–1.73) |
| Perinatal mortality | 32 (2.6) | 7 (3.4) | 1.33 (0.75–1.72) | 1.21 (0.80–1.85) |
| LOS >3 days | 189 (15.6) | 33 (15.9) | 1.01 (0.83–1.24) | 1.06 (0.83–1.34) |
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; FGR, fetal growth restriction; LOS, length of stay; NICU, neonatal intensive care unit; SGA, small for gestational age. OR and 95% CI are presented. Post hoc analysis controlling for maternal age, race, parity, BMI class and no prenatal care; aOR and 95% CI are presented. For the outcomes NICU admission, perinatal mortality and neonatal length of stay >3 days model also included gestational age at delivery and presence of fetal anomaly. Preterm delivery defined as <37 weeks.
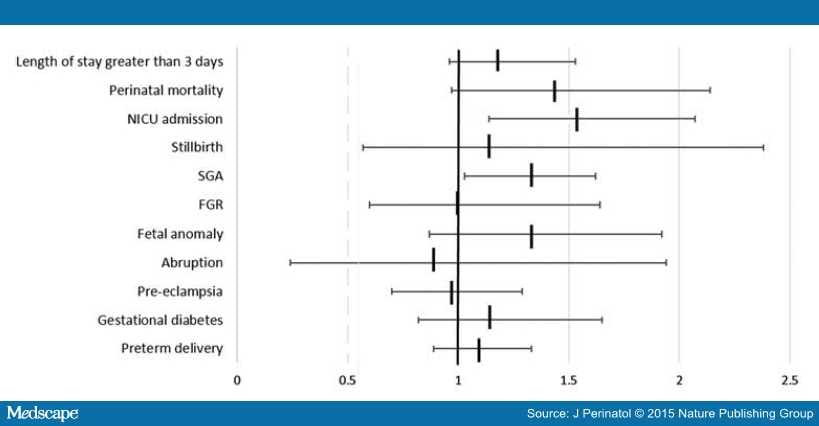 Figure 1.
Figure 1.
Adjusted odds ratio for selected obstetrical and neonatal outcomes comparing women who use marijuana with those who do not in non-tobacco users. Adjusted odds ratio and 95% confidence intervals are presented. Regression model inclusive of maternal age, race parity, BMI class and no prenatal care. For NICU admission and neonatal length of stay >3 days, model also includes gestational age at delivery. FGR, fetal growth restriction; NICU, neonatal intensive care unit; SGA, small for gestational age.
Discussion
We conducted a study to evaluate associations between marijuana use in pregnancy and major adverse obstetrical and neonatal outcomes and found a 30% increase in SGA and a 54% increase in NICU admission in women who used marijuana but were non-smokers. We did not find significant increases in these outcomes in women who also smoked tobacco. Prior studies on the association between marijuana use and SGA have been conflicting.[5,8] Tennes[8] analyzed a cohort of 756 pregnant women with reported marijuana use during pregnancy. When controlled for tobacco use, marijuana was not associated with poor fetal growth. Other more recent studies have found increases in the risk of lower birth weights with maternal marijuana use.[6,7,9] A key difference in our study’s methodology from these prior reports was stratification for concurrent tobacco use, not solely adjustment for this important confounder in post hoc analysis. We opted to design our study in this manner given maternal tobacco use is both strongly tied to marijuana use and to each of these outcomes. A meta-analysis evaluated the relationship between marijuana use and low birth weight and found an association with low birth weight if used frequently, but higher birth weight if not used frequently, and therefore the quantity of marijuana is likely a contributing factor, which our study was not able to evaluate.[11] Of note, our rate of prenatally diagnosed FGR was both low (2.8% in nonusers and 3.9% in marijuana users, P=0.24) and not significantly higher in marijuana users versus non-smokers (aOR 0.99 (95% CI 0.60 to 1.64)) and (aOR 1.23 (95% CI 0.86 to 1.76)) in smokers, indicating an overall low rate of detection of impaired fetal growth even in these higher risk patients.
We also found an increased rate of NICU admission in non-smokers, which has not been previously reported, as well as a concerning trend toward increased prolonged neonatal hospitalization and perinatal mortality that did not reach statistical significance. Finally, neonates born to mothers who used marijuana during the pregnancy had a higher rate of prolonged hospitalization over 3 days. All of the above associations were not found in tobacco users, which may be because these newborns are already at increased risk given tobacco use, and marijuana does not significantly marginalize this risk. In addition, we included 1423 women who used tobacco in our analysis, and 5045 women who were not tobacco users, and therefore had more limited power to establish an effect in all outcomes. Variance in methods for controlling for concurrent tobacco use may also help to explain discordancy in the literature. Varner et al.[4] reported an association between tetrahydrocannabinol use and stillbirth, however, they did not differentiate marijuana users from marijuana/tobacco users, and note ‘more research is needed to investigate the interaction of tetrahydrocannabinolic acid and cigarette smoking’. Fergusson[9] in a study of over 12 000 women did not find an increase in perinatal death. Our study did not find an increase in the rate of stillbirth alone, however our study was not powered to detect a difference in this rare outcome. We did not find an association between marijuana use and preterm delivery, an outcome that has conflicting evidence in the literature.[5–7] We believe our improved ability to exclude polysubstance use, which likely confounds risk for premature delivery, may explain this difference in our findings from prior reports.
Strengths of our study include the large number of women included in our analysis and our ability to control the confounding medical and social factors, such as race, obesity and lack of adequate prenatal care. In addition, maternal exposure data were taken directly from the medical record and based upon a combination of self report and toxicology screen results, and may be more accurate as opposed to reliance on self reporting in birth certificate records. Our rates of marijuana use are comparable to those generally reported and therefore we likely had reasonable ascertainment of use from the medical record. However, we did not perform universal drug screening and therefore women who did not admit to use and did not have other risk factors such as poor prenatal care and/or social or medical risk factors for drug use that would lead to toxicology screening by protocol may have erroneously been classified as nonusers.
Our study was not designed to determine dose-related effects and patients were characterized simply as ‘users’ or ‘nonusers’, although it is physiologically plausible that there may be differences in outcomes based upon increased use. It should also be noted that our center is a high-risk academic center with a large referral base, and as such our rates of exposures and outcomes may vary from other regions. Finally, we only report on outcomes to neonatal discharge, and there is consistent data that marijuana exposure during pregnancy and breastfeeding contributes to long-term cognitive, gross motor and neurodevelopmental impairments in the off-spring of these women which should be included in prenatal counseling of users.[12–17] A recent study by Goldschmidt et al.[16] examining school achievement assessed with the Wechsler Individual Achievement Test Screener in 14 year olds whose mothers had been recruited during their pregnancy for participation found higher rates of deficits in intelligence test performance at age 6 years, attention problems and depression symptoms at age 10 years, and overall negative effects of pregnancy marijuana exposure on adolescent achievement.
Our study was intended to be a general assessment of the associations between marijuana use in pregnancy and various obstetrical and neonatal outcomes. Therefore, we did not have sufficient power to draw conclusions for all the outcome variables studied, including perinatal mortality, but instead our goal is to describe trends and strengths of associations with various complications of pregnancy when there is maternal marijuana use. In addition, there are other confounders difficult to account for in our model such as poor socioeconomic status, educational level, nutritional deficiencies and so on that may be unaccounted for in our analysis. We hope that this may direct further study of the complications of marijuana exposure in pregnancy, especially as political and social changes evolve that could increase the rate of this exposure in pregnant women.
References
- Governing: The States and Localities. Available at http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html (retrieved on 20 February 2015).
- Drug Enforcement Agency: Dangers and Consequences of Marijuana Use. Available at DEA www.dea.gov/docs/dangers-consequences-marijuana-abuse.pdf (retrieved on 20 February 2015).
- Soto E, Bahado-Singh R. Fetal abnormal growth associated with substance abuse. Clin Obstet Gynecol 2013; 56(1): 142–153.
- Varner MW, Silver RM, Rowland Hogue CJ, Willinger M, Parker CB, Thorsten VR et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol 2014; 123(1): 113–125.
- Lee MJ. Marijuana and tobacco use in pregnancy. Obstet Gynecol Clin North Am 1998; 25(1): 65–83.
- Witter FR, Niebyl JR. Marijuana use in pregnancy and pregnancy outcome. Am J Perinatol 1990; 7(1): 36–38.
- Suarel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG 2014; 121: 971–977.
- Tennes K. Marijuana: prenatal and postnatal exposure in the human. NIDA Res Monogr Ser 1985; 59: 48–60.
- Fergusson DM. Maternal use of cannabis and pregnancy outcome. BJOG 2002; 109(1): 21–27.
- Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381.
- English DR, Holse GK, Milne E, Holman CD, Bower CI. Maternal cannabis use and birth weight: a meta-analysis. Addiction 1997; 92(11): 1553–1560.
- Jaques SC, Kingsbury A, Henshcke P, Chomchai C, Clews S, Falconer J et al. Cannabis, the pregnant woman and her child: weeding out the myths. J Perinatol 2014; 34(6): 417–424.
- Brown HL, Graves CR. Smoking and marijuana use in pregnancy. Clin Obstet Gynecol 2013; 56(1): 107–113.
- Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 2002; 110(6): 1182–1192.
- Chandler LS, Richardson GA, Gallagher JD, Day NL. Prenatal exposure to alcohol and marijuana: effects on motor development of preschool children. Alcohol Clin Exp Res 1996; 20(3): 455–461.
- Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL. School achievement in 14-year old youths prenatally exposed to marijuana. Neurotoxicol Teratol 2012; 34(1): 161–167.
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16- year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2003; 25(4): 427–436.
Acknowledgments
Study data were collected and managed with REDCap software, which is hosted at Cincinnati Children’s Hospital Medical Center under the Center for Clinical and Translational Science and Training grant support (UL1-RR026314-01 NCRR/NIH). REDCap is a secure, web-based application that was designed to support data capture for research studies to provide (1) an intuitive interface for validated data entry, (2) audit trails for tracing data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for importing data from external sources.